Nature Conservation 2022 — 25. 5. 2022 — On Nature in the Czech Republic — Print article in pdf
Soil as a Biodiversity Hotspot, Namely that of Small Soil Arthropods

When presenting examples of particularly species-rich ecosystems, we will most probably name tropical coral reefs, tropical gallery forests or selected types of tropical rainforests. To visit these ecosystems, one must travel a long journey far outside of homeland, and even beyond borders of our continent. Still, a comparably species-rich ecosystem can be found, even in our conditions – yet escaping broader awareness when speaking about biodiversity. This is perhaps caused by simple fact, that thousands of species inhabiting this ecosystem could only rarely be observed by the naked eye, as they are mostly of the microscopic size. Indeed, healthy, and well-functioning soil – in our conditions mostly soil of broad-leaved deciduous or mixed forests – is a real biodiversity hotspot reachable just “beyond our courtyards” or next door. Presence of the particular species, overall species richness and relative numbers of soil organisms serves at the same time as great indicator of soil conditions, and often also of the quality of respective above-ground ecosystem at the place.
Functions are the key
Soil and soil surface represent in terrestrial ecosystems a space securing one of the principal eco- system services: gradual decomposition of dead organic matter. Only a small part is not finally com- pletely decomposed to original components – mineral nutrients and carbon dioxide – and enters complex chemical transformations leading into creation of humic substances with the very variable and complicated structure. Despite the fact, that it includes only a fragment of overall volume of dead organic matter, the process is spatial and rather constant in healthy soils. This results in fixation and accumulation of atmospheric carbon in the soil, where it can be stored for variable, but sometimes very long time, making it in principle similar to that of fossil fuels. Soil is by far the largest terrestrial sink on the Earth, storing around 2300 Gt of carbon (Stockmann et al. 2013), about 2.3 times more than atmosphere and 3.5 times more than in living plant biomass.
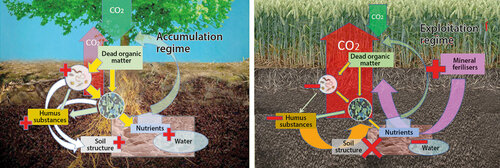
Figure 1 Simplified scheme of soil ecosystem functioning. Under normal conditions, dead organic matter in soil is decomposed by soil decomposers (bacteria, fungi, soil fauna) back to nutrients, part of decomposed matter however remains in the soil as complex humus substances. Thanks to the process, carbon is accumulated in soil and at the same time a complex soil structure is built and maintained. Soil structure is a precondition for effective water holding capacity and capturing of nutrients in the system (this regime is called “accumulation regime”) [left]. In intensively utilised soils, the entry of dead organic matter may be dramatically lowered, and plant nutrition is served by addition of (soluble) mineral fertilisers. Mineral fertilisers cannot be used by soil organisms as a source of energy, therefore organic deposits in the soil are more utilised and humus substances are more intensively decomposed (“the soil is feeding itself”). Numbers of soil organisms are declining, soil structure is collapsing and subsequently also the water and nutrient holding capacity is declining, too. Consequently, amounts of released carbon dioxide from the soil excess amounts stored in the system (exploitation regime). Red plusses and minuses indicate increase or decrease, size of the arrows indicates schematically intensity or volume of the particular process. © Ladislav Miko
Therefore, disturbances of soil decomposition processes – such as warming, increased oxidation or limitation of dead organic matter input – may cause increased decomposition, mobilisation and release of stored carbon, resulting ultimately in transformation of soil sink into atmospheric carbon dioxide (or other greenhouse gas) source (Fig. 1). Farming, for exam- ple, has resulted in emission of approx. 116 Gt of carbon into atmosphere since its beginning (Sandemann et al. 2017), with more substantial part of it having been released in the last several decades by intensive farming (data refer to 1998, so at present the values are even higher).
Within the ecosystem, however, the nutrient cycling is a key service of organic matter decomposition. Dead organic matter is still relatively energy-rich, which is the main attractant for soil organisms involved in decomposition. The most significant role in decomposition, leading up to mineralisation to original components (nutrients), is played by primary decomposers – soil bacteria (including archea) and fungi, called sometimes historically “soil microflora”. The species richness of these organisms is enormous and has mostly been unknown – partly because it is very difficult to study without DNA analysis or specific cultivation approaches.
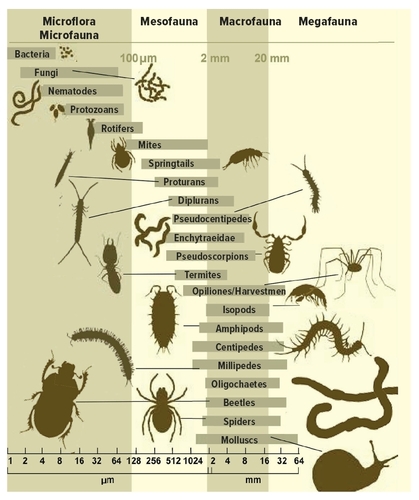
Activities of the primary decomposers in soil are broadly “assisted” by numerous representatives of soil fauna. Their abilities and functions in soil ecosystem are influenced by multiple of factors such as availability of specific enzymes but also relative size and ability to actively penetrate the soil environment, speed and rate of movement and other specific adaptations. Some of these traits seem to be functionally related to size (or diameter) of the body and therefore reflected in usual size categories of soil fauna (Fig. 2). The way of exploitation of resources in decomposition processes is a base for functional categorisation of soil fauna (Šantrůčková et al. 2018).
Figure 2 Categorisation of soil organisms based on effective diameter of the body, which is related to functioning in the soil ecosystem. Source: Miko et al. (2019). © Ladislav Miko
Soil engineers (earthworms, but also termites, ants or insect larvae) create soil burrows and spaces, thus securing a distribution of dead organic matter across the soil profile, as a prerequisite for activities other soil organisms. Large and medium sized species (millipedes, isopods, insect larvae, enchytraeids or potworms) are feeding on organic debris, causing its fragmentation and transformation into structured excrements (faecal pellets, etc.); they represent therefore the functional group (a guild) of litter transformers. Smaller species (microarthropods, mostly mites and springtails, and diverse group of nematodes) are feeding dominantly on fungi and bacteria. Some of them are adapted to penetrate the cells and fungal hyphae to suck their content, but mostly they are not able to separate microflora from the substrate (organic debris), so they consume both together, creating a functional group of microbial feeders and browsers. This broad group, however, contributes also to fragmentation of organic matter, leading ultimately to creation of increased surface available for primary decomposers activities, and generally to acceleration of decomposition. Movements of soil fauna in litter and organic debris of soil result in efficient mixing of organic fragments with mineral soil particles (bioturbation). Soil microfauna represented by the smallest-bodied set of species (protists, rotifers, tardigrades, many nematodes) inhabits usually water-filled micro-spaces or water films on soil particles surface, often feeding by filtration of bacteria or other small species (predation, in principle), or utilise dissolved energy-rich substances (osmotrophic).
Broad array of species dependent on organic matter and primary decomposers on its surfaces obviously becomes a broad potential source of prey for cascades of soil predators. Many of them can utilise also freshly dead bodies (necrophagy). Other set of species in soil may feed on excrements of other species (coprophagy).
Regular input of dead organic matter (leaf litter or other dead parts of plant bodies, excrements, carcasses, etc.) is a relative surplus of feed, and contributes (together with products of decomposition process) to creation of highly complex soil structure. As a result, soil organisms are not necessarily competing for space and energy, which allows for co-existence of extreme high number of species and consequently for very high biodiversity of the soil system. Principally important is also the phenomenon of functional redundancy (Setala et al. 2005) where many species can overlap in their way of resource utilisation (e.g. food preferences).
This, at first sight unnecessary, redundancy is in fact an extremely useful “insurance” securing functioning of the system even in case of dramatic reduction in species composition in the soil (portfolio effect). At the same time, it can partly overshadow worsening of soil conditions, for example biodiversity decline, because in terms of ecosystem functions it may not be visible. As we can have an impression, that soil “still works” as usual, we may not notice that soil biodiversity declines and soil may irreversibly undergo degradation.
Probably the easiest way to observe and document the species explosion in the soil ecosystem is to study soil mesofauna, dominated usually by springtails (Collembola) and mites (Acarina), represented in particular by mossmites, or oribatids (Oribatida). The species diversity and bioindication capacity of soil fauna is further demonstrated using example of oribatid mites.
Species richness and abundance
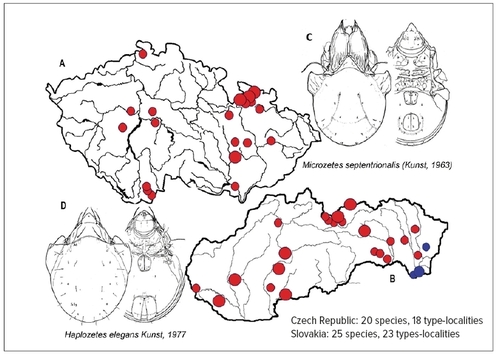
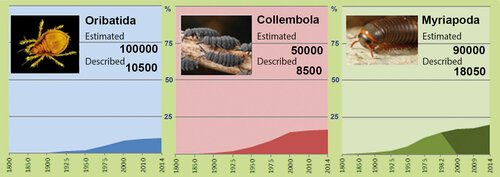
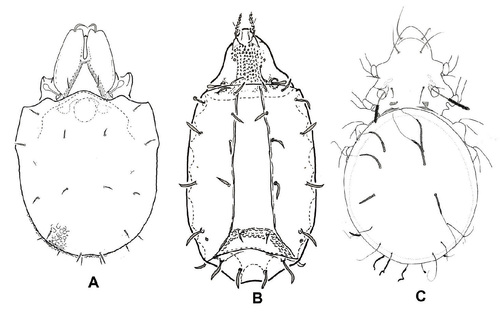
New species of soil microarthropods are regularly found and described from the territory of the Czech Republic and Slovakia, though the Central Europe is among the most studied are- as of the planet in that respect. Type-localities of many oribatid species, located there (Fig. 3), should stay preserved in their natural state – but many of them have not even been included in the system of protected areas. Regular discovery of new species (or findings of species known from other – distant – localities only) in basically any territory searched more systematically and in detail, demonstrates first extraordinary species richness of soil microarthropods and second still very fragmented knowledge of it. According to the recent revision, 539 species of oribatids were confirmed in the Czech Republic’s territory (Miko 2006). The number of species of other mite groups and springtails, even if slightly lower, is also counted in hundreds. Globally, the number of described species has also been fast growing – today about 10,500 species of oribatids and over 8,500 species of springtails (Orgiazzi et al. 2016) – still representing only a fragment of the estimated number of species (Fig. 4). Interestingly, the number of known species in the Czech Republic is not much different compared to other similarly explored countries (Jeffery et al. 2010).
Figure 3 Many type-localities of newly described oribatid mite species can be found on the territory of the Czech Republic (A) and Slovakia (B). Only part of them is included in protected areas. Species (C, D) are examples of the species described from the territory of Czech Republic and Slovakia. © Ladislav Miko
Figure 4 Increase in numbers of described (known) species as a part of overall estimated numbers of species in three soil fauna groups. All charts are demonstrating poor knowledge of overall biodiversity of soil organisms. © Ladislav Miko
Soil microarthropods can be found almost everywhere – even in dust on the road margins. Moreover, in habitats with intensive human impact there are mostly represented by few generalists, broadly tolerant species. Number and share of these (eurytopic) species in overall fauna may therefore indicate the quality, level of preservation or succession stage of the studied habitats. Similar information can also be provided by abundance of soil microarthropods. In general, increased intensity of human impacts not only simplifies the species composition, but also causes decrease in overall abundance. However, abundance is dependent on many other factors, such as overall amount of available dead organic matter, humidity, temperature, pH value or salinity of the soil – and this needs to be considered when assessing the anthropogenic impact. In mountain forest soils with acid deposits of humus of modertype, for example, the abundance of oribatid mites may reach 250–300 thousand of individuals per square meter (exceptionally over 1 million), while even in the best quality farmed soil it hardly reaches several tens of thousands of individuals. In degraded, intensively farmed soils the abundance reaching few thousands or hundreds of individuals per square meter is not exceptional, and even “empty” samples may be found. This indicates a long-term loss of quality in the environment – and similar bioindication may be used to measure the restoration of soil quality parameters, e.g. in case of organic farming.
Relics and specialised species as indicators of high value ecosystems
From a point of view of nature conservation, soil microarthropods are not just remarkable due to their high species richness. Many species are quite narrowly adapted and specialised and at the same time able to survive for quite a long period of time at the particular site, even if original habitat has been significantly fragmented or altered. This allows to find which habitats were present at the given site, and together with analysis of potential vegetation to learn about history of a given place. Presence of relic species, or, from another perspective, species arriving due to changed climate and consequently induced habitat transformation, can provide useful information about ongoing changes. Alpine and sub-alpine meadows of the highest mountains, mountain peat-bogs or fragments of primeval forests are often characterised by the presence of boreal or boreo-montane species. The presence of species known primarily from high alpine European mountains (the Alps, the Carpathians) indicate the extraordinary character of Czech mountains, in spite of their relatively moderate elevation (Fig. 5).
Figure 5 Rare, highly specialised or relic species of oribatid mites may indicate the extraordinary importance of their habitats – sub-alpine mountain areas with frost-modulated soils in the Krkonoše/Giant Mts. (A, B) or cave systems of the Moravský kras/Moravian Karst (C). A – Unduloribates undulates (Berlese, 1914); B – Camisia (Ensicamisia) solhoeyi Colloff, 1993; C – Metabelbella clavigera (Willmann, 1954). Source: A, B Weigmann (2006), C original © P. Ľuptáčik
Table 1 Known and estimated species richness in the selected soil organism groups
|
group/taxon |
known species |
estimated species |
|
Earthworms (Oligochaeta Lumbricoidea) – macrofauna |
7,000 |
30,000 |
|
Myriapods – macrofauna |
18,050 |
90,000 |
|
Ants (Hymenoptera Formicoidea) – macrofauna |
14,000 |
25,000-30,000 |
|
Mites (Acari), soil living – mesofauna |
55,000 |
100,000-150,000 |
|
Springtails (Collembola) – mesofana |
8,000 |
5,0000 |
|
Nematodes – microfauna |
25,000 |
1 to 10 million |
|
Protists – microfauna |
21,000 |
7 to 70 million |
|
Fungi – microflora, primary decomposers |
97,000 |
1.5 to 51 million |
|
Bacteria and Archaea – microflora, primary decomposers |
15,000 |
over 1 million |
|
Total (many groups not included) |
260,050 |
approx..11 to 132 million |
Increased presence of xerophilous, heliophilous or termophilous species, often originating from the Mediterranean region and originally known only from southernmost sites of the Czech Republic, documented from several new, more northern localities (Fig. 6) may indicate direct or indirect climate change impact in Europe as well as in the Czech Republic. Presence of particular oribatid species may show the relic character of the whole habitat, this was the base for listing of several species in the Red List of Invertebrates of the Czech Republic (Miko 2017) as umbrella species of their soils. Also caves and generally karstic areas include very specific, and still mostly unknown microarthropod fauna, at least partly derived from species living in deep soil layers and crevices in weathering bedrock. Even if in Central Europe the species richness is apparently lower than in karst areas of the Balkan Peninsula (Romania, Slovenia, Croatia), even there some new and locally specific species have been discovered (Fig. 5). Detailed research in these areas may still bring some surprises – for example showing genetic heterogeneity (and hidden biodiversity) of cave populations, documenting colonisation of underground space and isolation or vice versa interconnections of their populations.
Figure 6 Oribatid mites may indicate habitat quality and level of anthropogenic pressure e.g. in open (non-forested) habitats. Tectocepheus velatus (Michael, 1880) (A) belongs to the most tolerant species and occurs almost anywhere, in soils with high level of disturbances may be often the single present species. – Peloptulus phaenotus (C. L. Koch, 1844) (B) is a common species of dry and warmer meadow habitats, could be found also outside of well-preserved steppic and forest-steppe habitats. – Licnodamaeus pulcherrimus (Paoli, 1908) (C) occurs in a majority of well-preserved xerotherm grasslands and steppes. – Passalozetes africanus Grandjean, 1932 (D) indicates well preserved fragemnts of indigenous steppes and forest-steppes. – Eubelba sculpta (Mihelčič, 1957) (E) has been discovered on the territory of the Czech Republic only very recently, and represents rare species of dry grasslands on sandy soils. In the best preserved steppic habitats and grasslands on sandy soils, specialised predatory mites from the family Caeculidae (belonging to Trombidiformes, Prostigmata), such as Allocaeculus sandbergensis Mangová, Krumpál et Ľuptáčik, 2014 (F), described recently from Devínska Kobyla Hill (Bratislava, Slovakia) with highly adapted raptorial front pair of legs are rarely present. © Ladislav Miko
Fossil and subfossil record, importance for paleobiology
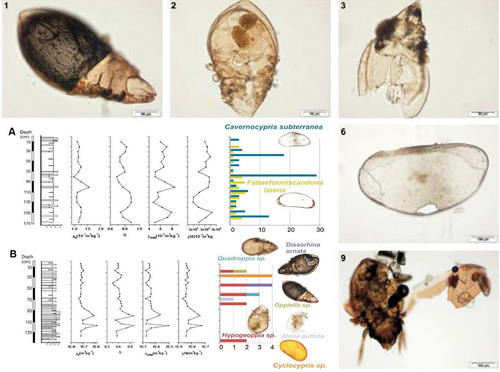
Body of many soil microarthropod species is soft and not heavily sclerotized, so that dead individuals are either fast consumed or decom- posed. Species with a robust, chitinized body – such as oribatid mites, but also other mites (Mesostigmata, some predatory Prostigmata) and exceptionally some species from other groups (e.g. pauropods) – may sustain for a long time in the particular conditions of peat bogs or in clastic sediments allowing their determination. Study on communities or particular species preserved this way in well-dated fossil or sub-fossil layers (Fig. 7) provides an useful proxy information about past environmental conditions at the site, or (in case of alluvial sediments) in the vicinity upstream. The gathered information may serve as a good background for modelling and forecasting future developments, e.g. again in relation to climate change impacts. This approach already allowed to proof changes in soil microarthropod communities with changed paleoclimate, confirmed or completed available information about changes in vegetation, or even indicated significant impact of human settlements and soil cultivation on ecosystems already in time of very extensive use (Moldovan et al. 2011, 2016).
Figure 7 Fossil remnants of oribatids (1-3, 9), together with other species with sclerotised body cover (such as Ostracoda – 6) can be used in combination with different dating methods (A, B) to reconstruct ecological conditions in the near and distant past. Source: Moldovan et al (2016), adapted. © Ladislav Miko
The option to study (sub-)fossil material allows also for quite different, phylogenetic and taxonomic studies. The oldest known and described fossil oribatids were found in Devonian sediments (Norton et al. 1988), further findings from the Carboniferous and from the Deuterozoic periods allow to better understand evolutionary trends in the whole group of chelicerates. The very fact, that Devonian oribatids closely resemble some of the recent species (Fig. 8), indicates how different may be the evolutionary pathways in differing soil conditions, and supposes that evolution of different arthropods may apparently undergo in “pockets” – sometimes very slowly and sometimes very fast and with highly intensive species radiation. At the same time, we can say that the so called “living fossils” – such as well-known Latimeria fish – are not just phenomenon occurring in distant deep ocean waters, but may be, with a bit of luck, observed even in soil of our own gardens.
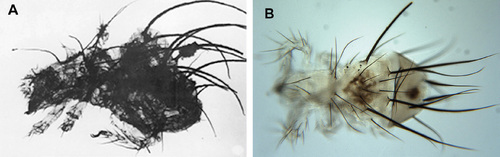
Figure 8 Findings of oribatid fossils suggest that some of the common species of the present are in fact a living fossil. (A) Fossil species Devonacarus sellnicki Norton et al., 1988 from Devonian sediments in the U.S.A. is very similar to recent species Paleacarus hystricinus Tragardh, 1932 (B), which can also be found in the territory of the Czech Republic. © Ladislav Miko
Soil health and ecosystem restoration
Traits and characters of soil microarthropods, mentioned above, are clearly very useful as practical indicators in biodiversity studies and nature conservation in general. They can be used to indicate a level of anthropogenic disturbance or soil degradation (e.g. when assessing the quality of agricultural soils), or as general indication of soil environment quality and function in assessment of planned interventions (for example as a part of EIA studies). Survey of soil fauna was used e. g. in deciding about alternatives of highway building across the České Středohoří/Central Bohemian Uplands Protected Landscape Area (even if ultimately other – political – interests prevailed, information from survey still helps in managing details, which are not available or achievable on the basis of other environmental studies and surveys). Studies of soil mesofauna can document the value of non-intervention management in Specially Protected Areas, preserving of soil quality and functionality in natural forests, or – as in the case of the Na Plachtě Nature Monument in the city of Hradec Králové (east Bohemia) – it may prove high biodiversity value of areas intended for further development. Species richness, together with presence of many rare species, may document unique quality of some Czech Specially Protected Areas – the Velká Kotlina/Grand Fold in the Hrubý Jeseník Mts., Nature Reserves of the Beskydy Mts., peat-bogs of the Šumava/Bohemian Forest Mts. National Park or habitats in the Krkonoše/ Giant Mts. National Park may serve as good examples (Miko 1986, 2014, Starý 1988, 1993).
Recent studies have documented also usefulness of soil microarthropod communities as indicators of succession and ecosystem restoration. Studies on bare soil deposits/spoil heaps from surface open-pit brown coal mining in northern Bohemia show that spontaneous succession may deliver higher biological and ecological value including sustainability parameters, than often very costly artificial man-made active remediation approaches (Frouz et al. 2008). Development of soil microarthropod communi- ties after targeted management measures – e.g. in heathland or peat-bog restoration projects – may help to check that restored ecosystems are brought after human disturbances back to more natural, spontaneous trajectories, which are desirable from the nature and biodiversity conservation point of view (Frouz et al. 2009).
Missing expertise and further research needs
From presented – and still incomplete – overview of possibilities, offered by soil organisms’ studies, it is evident that their importance for applied ecology, biodiversity conservation and nature protection and management, but also for agricultural practice or environmental assessment approaches may be significant. The main obstacle at present is the lack of capacity, missing practical experience and absence of the agreed common methodologies, with linked absence of broadly accessible publication sources. Number of experts for particular groups of soil organisms, perhaps with exemption of microbiological studies, can be counted on the fingers of single hand, and this situation does not differ significantly from that in other European countries or even globally. Interest to study soil organisms and to apply study results in practice has been growing only very slowly. Recently published of Czech-language textbooks and practical manuals of soil biology and ecology (Šantrůčková et al. 2018; Miko et al. 2019; Šimek et al. 2019) could hopefully boost this interest and allow for far broader utilisation of opportunities, offered by study on soil fauna. ■
List of referencies
Frouz, J. et al. (10 spoluautorů)(2008): Interactions between soil development, vegetation and soil fauna during spontaneous succession in post mining sites. European Journal of soil biology 44 (2008): 109-121
Frouz, J. et al. (7 spoluautorů)(2009): The effect of topsoil removal in restored heathland on soil fauna, topsoil microstructure, and cellulose decomposition: implications for ecosystem restoration. Biodiversity Conservation 18: 3963-3978
Jeffery, S. et al. (10 spolueditorů) (2010): European atlas of soil biodiversity. European Commission, Publication Office of the European Union, Luxembourg, 128 pp.
Miko, L. (1986): Příspěvek k poznání fauny pancířníků (Acari, Oribatei) Hrubého Jeseníku. Časopis Slezského Musea Opava (A), 35: 273-283
Miko, L. (2014): History of oribatid studies (Acarina, Oribatida) in the Krkonoše National Park (the Giant Mountains, Czech Republic), with a revised checklist of all known species of Giant Mountains. Opera Corcontica 50/S: 143-164
Miko, L. (2016): Oribatid mites (Acarina: Oribatida) of the Czech Republic. Revised check-list with a proposal for Czech oribatid nomenclature. Klapalekiana, 52 (Suppl.):1-302.
Miko L. (2017): Oribatida (pancířníci). – In: Hejda, R., Farkač, J. & Chobot, K. (eds.), Červený seznam ohrožených druhů České republiky. Bezobratlí. (Red List of Threatened Species of the Czech Republic. Invertebrates). Příroda, Praha, 36: 83-85.
Miko, L.; Šantrůčková, H.; Záhora, J.; Máchal, A. (2019): Život v půdě. Příručka pro začínající půdní biology. Lipka, 240 pp.
Moldovan, O. et al. (6 spoluautorů)(2016): Fossil invertebrates in cave sediments and paleoenvironmental assessments – a study of four cave sites from Romanian Carpathians. Biogeosciences, 13, 483-497
Moldovan, O. et al. (7 spoluautorů)(2011): Invertebrate fossils from cave sediments: a new proxy for pre-Quaternary palaeoenvironments
Norton, R. A., Bonamo, P. M., Grierson, J. D., Shear, W. A. (1988): Oribatid mite fossils from a terrestrial devonian deposit near Gilboa, New York. Journal of Paleontology, 62, 2: 259-269
Orgiazzi, A. et al. (27 spolueditorů)(2016): Global soil biodiversity atlas. European Commission, Publication Office of the European Union, Luxembourg, 176 pp.
Sanderman, J., Hengl, T., Fiske, G. (2017): . PNAS 114(36): 9575–9580. doi:10.1073/pnas.1706103114
Setälä, H., Berg, M., & Jones, T. (2005). Trophic structure and functional redundancy in soil communities. In R. Bardgett, M. Usher, & D. Hopkins (Eds.), Biological Diversity and Function in Soils (Ecological Reviews, pp. 236-249). Cambridge: Cambridge University Press. doi:10.1017/CBO9780511541926.014
Starý, J. (1993): Pancířníci (Acari: Oribatida) Moravskoslezských Beskyd, Česká Repubika. Časopis Slezského Musea Opava (A), 42: 259-266
Starý, J. (1988): Pancířníci (Acari: Oribatida) některých vrchovišť na Šumavě, Jižní Čechy. Sborník Jihočeského muzea v Českých Budějovicích, Přírodní vědy, 28: 99-107
Stockmann, U. et al. (2013): The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agriculture, Ecosystems and Environment 164 (2013): 80-99
Šantrůčková, H.; Kaštovská, E.; Bárta, J.; Miko, L.; Tajovský, K. (2018): Ekologie půdy. Episteme, České Budějovice, 260 pp.
Šimek, M. et al. (38 autorů)(2019): Živá půda. Academia, 2 svazky, 789 pp.
Weigmann, G. (2006): Hornmilben (Oribatida). Die Tierwelt Deutschlands. Goecke & Evers, Keltern. 520 pp.